


Asymchem offers a comprehensive stability assessment and testing services to ensure the quality, efficacy and compliance of our client's compounds. Stability studies assess how compounds are affected over time by environmental factors, such as temperature, humidity and light, and are essential for establishing appropriate packaging, storage and transportation conditions, as well as determining shelf life.
Stability Capabilities

In addition, we can complement our development services with comprehensive range of stress testing and forced degradation studies. These stress tests are designed to identify potential degradation of materials under extreme or unusual conditions, helping to explore their inherent stability.
All stability studies are essential for providing data to support regulatory filings and ensure the compounds quality and compliance with regulatory standards across global markets. Utilising our state-of-the-art technology, alongside our in depth experience and knowledge, we can rapidly execute these studies from the initial planning, through to execution and assessment.
Stability Testing
Adhering to the guidelines specified in ICH Q1A, Q1B, and Q1E, Asymchem can conduct studies to generate real-time stability data, which can be used to determine use periods, shelf-life and support regulatory submissions.
Asymchem can also facilitate accelerated and intermediate storage conditions to assess the impact of short-term deviations from the labelled storage conditions, such as those that might occur during shipping.
Stability Chambers
Our stability chambers support all ICH conditions:
-20°C
5°C
25°C/60%RH
30°C/65%RH
40°C/75%RH
Photostability
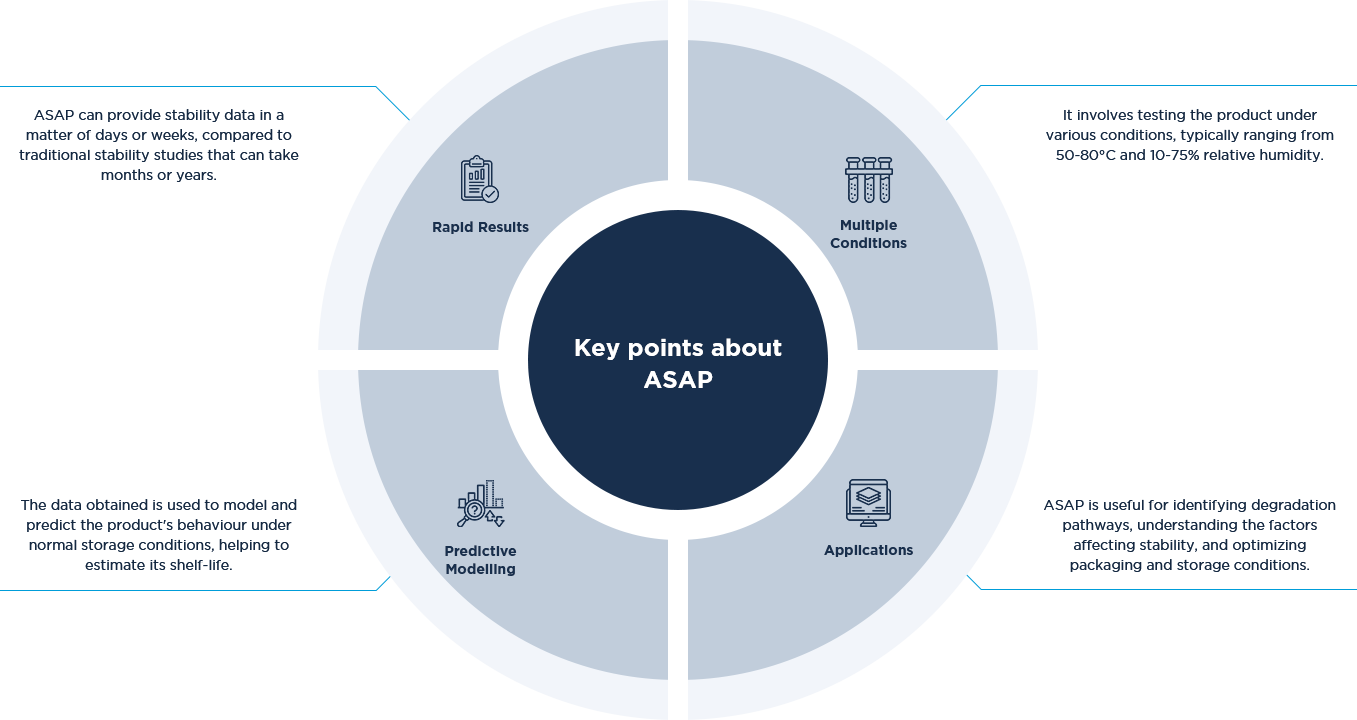
Accelerated Stability Assessment Program (ASAP)
An Accelerated Stability Assessment Program (ASAP) is a method used to quickly predict the shelf-life of a product by subjecting it to elevated stress conditions, such as higher temperatures and humidity levels. This approach is based on the principles of the humidity corrected Arrhenius equation, which relates the rate of chemical reactions to temperature and humidity. Asymchem experienced scientists work closely with our clients to design and implement these studies to provide meaningful stability data speedily.
Forced Degradation
At Asymchem, forced degradation studies are a key part of our analytical and method development workflow. Asymchem can accommodate a variety of experimental conditions, including hydrolytic degradation, oxidative degradation, photolytic degradation, and thermal degradation to provide the client with a comprehensive understanding of their compound.
Key learning outcomes of forced degradation studies
Establishing likely degradation pathways and products
Evaluating the stability of a molecule
Gaining insight into packaging and storage
Providing a foundation for the development and validation of stability-indicating analytical methods