Leading Analytical Excellence with Modern Technology and Innovation
Our integration of advanced in-silico modeling tools with sophisticated instrumentation, along with our years of experience and success, allows us to optimize and streamline the separation of key components. Our streamlined workflow allows us to achieve faster method development with increased knowledge of the design space, leading to robust analytical methods ready for commercial transfer. Our close collaboration with our clients allows us to develop methods that meet or exceed their requirements, faster.
Analytical Development Support
Analytical Method Development
Method Development Workflow

Method Validation
Asymchem provides comprehensive support for the validation of analytical test methods, providing expert guidance determining stage-appropriate validation and strategies. Our validation processes can be customized to suit the specific requirements of clients at any stage of development.
Method Validation and Verification
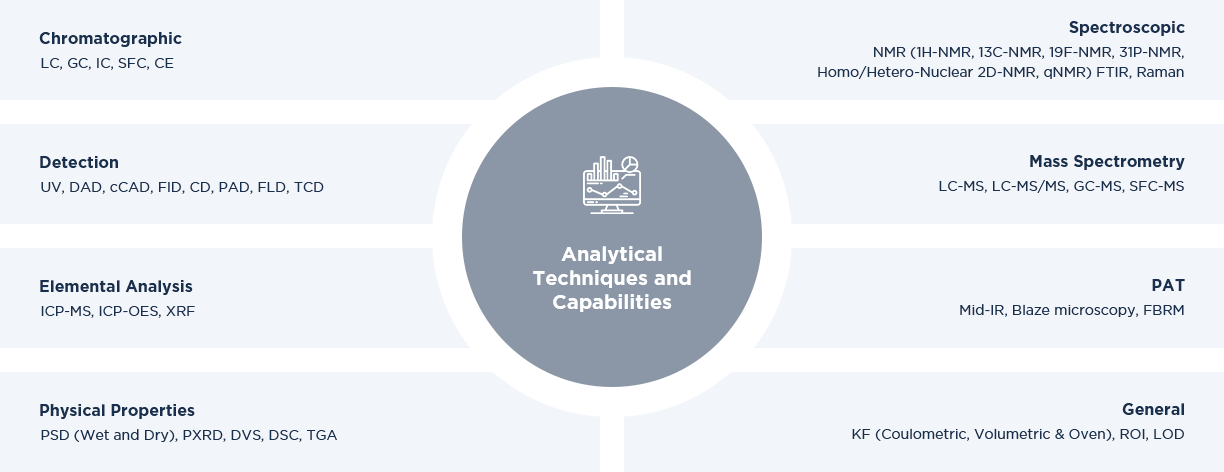
Analytical Techniques and Capabilities
At Asymchem, we have state-of-the-art laboratories equipped with a variety of sophisticated analytical equipment, to meet client’s needs and requirements. Our scientists are highly experienced and offer services in the development of a wide range of analytical methods including genotoxic impurities, elemental analysis, nitrosamines and identification of unknown compounds.
Analytical Techniques and Capabilities